-
High Light
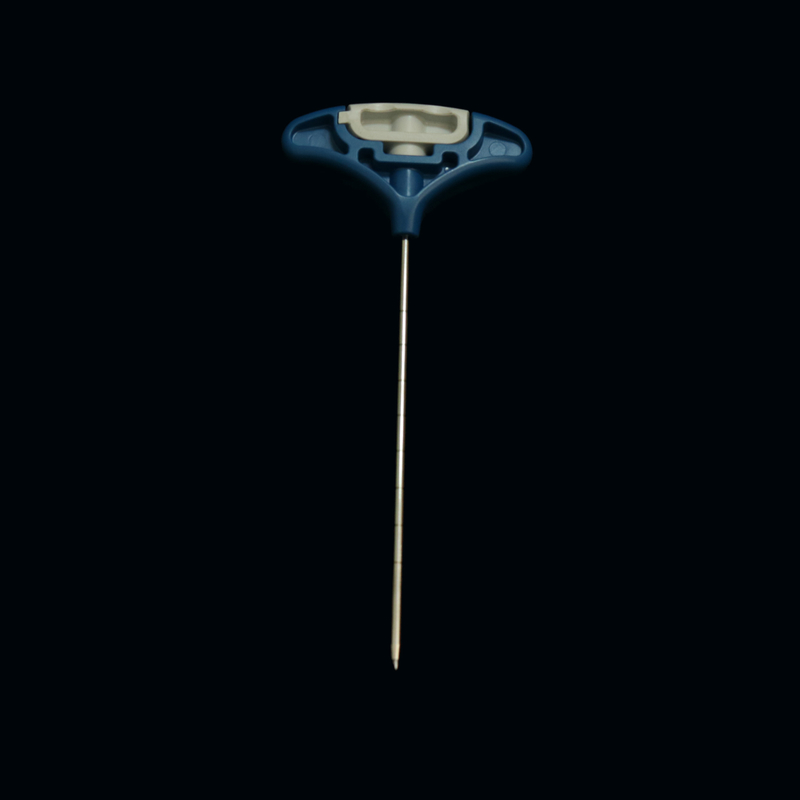
surgery tool kit
,surgical tool kit
-
Product NameKyphoplasty Tool Kit
-
Working Channel≥3.1/3.65mm
-
CertificationCE/ISO13485
-
Main MaterialStainless Steel, ABS
-
ColorBlue Or Customized
-
Instrument ClassificationClass II
-
Shelflife3 Years
-
TypeHand Tools
-
Place of OriginChina
-
Brand NameChangmei
-
CertificationCE
-
Model NumberKT-01-01
-
Minimum Order Quantity100sets
-
Price$80~100/set
-
Packaging DetailsCarton size: 420*360*375mm
-
Delivery Time15-30days
-
Payment TermsT/T
-
Supply Ability20000sets/year
Percutaneous Kyphoplasty Kit Stainless Steel / ABS Main Material
Medical Devices Percutaneous Orthopedic Vertebroplasty Tool Kit
Intended use:
The bone filler is developed on the basis of the existing vertebroplasty instruments and can be used together as a new type of vertebroplasty for the treatment of vertebral compression fractures. The container and the introducer are introduced through the working channel, and the spiral syringe injects the bone filling material into the filling container to expand the container, thereby raising the end plate, restoring the height of the vertebral body, and reducing the pain. Can reduce the rate of bone cement leakage. The osteophyte container consists of an instrument introduction and a container. Introduction instruments include: introduction tube, guide pin, push rod. The introduction tube has two forms, single tube and double tube. Double tube type outer casing. There are two types of containers, one is a single layer and the other is a double layer.
Benefits:
The operation is simple and convenient, the operation time is short, the postoperative recovery is fast, the effect is obvious, the pain can be relieved immediately, and the strength of the injured vertebral body is enhanced. The design of this set of products is small in diameter, and at the same time, the processing technology is improved, the percutaneous intervention is fast and effective, and the bone tissue guiding channel is established to effectively reduce the wound.
Specifications:
Kyphoplasty tool kit components list-8G
|
Serial number |
Name |
Model&Specification |
KT-00-01 |
KT-00-02 |
KT-00-03 |
KT-00-04 |
KT-00-16 |
KT-00-17 |
|
1 |
Puncture expandor |
KT-01-01 |
1 |
1 |
1 |
1 |
1 |
1 |
|
2 |
Puncture expandor module |
KT-02-01 |
1 |
X |
1 |
X |
2 |
1 |
|
3 |
Expandor device |
KT-03-01 |
2 |
2 |
1 |
1 |
X |
X |
|
4 |
Bone cement filling device |
KT-04-01 |
6 |
6 |
3 |
3 |
6 |
3 |
|
5 |
Bone drill |
KT-05-01 |
1 |
1 |
1 |
1 |
1 |
1 |
|
6 |
Guide wire |
KT-06-01 |
2 |
2 |
1 |
1 |
X |
X |
Kyphoplasty tool kit components list-11G
|
Serial number |
Name |
Model&Specification |
KT-00-05 |
KT-00-06 |
|
1 |
Puncture expandor module |
KT-07-01 |
2 |
1 |
|
2 |
Bone cement filling device |
KT-08-01 |
6 |
3 |
|
3 |
Bone drill |
KT-09-01 |
1 |
1 |
Why Choose Us
Jiangsu Changmei Medtech Co., Ltd. is mainly engaged in medical equipment products and production equipment design and development and manufacturing, in particular focus on the medical balloon catheter market, the company in the innovative balloon technology, production equipment and integrated manufacturing field closely follow the trend of the world, is committed to the medical balloon catheter in the case of domestic.
- With a modern standard workshop of 6000m2,among which,1600m2 are class 100000 clean rooms in compliance with YY0033;
- National class II and class III medical device production license;
- EN ISO 13485:2012 authentication certificate by TÜV SÜD;
- With numerous patents, Jiangsu Province Science and Technology of private enterprises;
- Experienced in interventional and endoscope equipment research and development of minimally invasive diagnosis and treatment;
- Our products have been in service for improving patients’ quality of life all over the world.
Packing & Delivery:
Packing:Dupont bag sterilized packaging, two layers plastic sealing, foam paper and box packaging.
Lead time:The goods in stock can be shipped within 7 days, while the goods for OEM or ODM services need about 30 working days to be shipped after receiving 100% prepayment.
FAQ
Q: Are you trading company or manufacturer ?
A: We are manufacturer located in Changzhou Jiangsu China,which could supply OEM&ODM services.
Q: How long is your delivery time ?
A: Generally it is 7 working days if the goods are in stock. Or it is 30-60 days if the goods are not in stock, it is according to quantity.
Q: Can you produce according to customers' design ?
A:Yes, we are professional manufacturer; OEM and ODM are both welcomed.
1)Silk print logo on the product;
2) Customized product housing;
3) Customized Color box;
4) Any your Idea on product we can help you to design and put it into production.
Q: Do you provide samples ? Is it free or extra ?
A: Yes, we could offer the sample but for the extra price.
Q: What is your terms of payment ?
A: T/T. If you have any question, Please contact with us.
![]()
![]()
![]()